
Journal Name: Molecular Neurodegeneration
Overview
Molecular neurodegeneration is a rapidly advancing field in neuroscience focused on the molecular and cellular mechanisms that underlie the progressive loss of structure and function of neurons. These processes are central to many neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and Amyotrophic Lateral Sclerosis (ALS). With global populations aging and neurodegenerative diseases on the rise, understanding molecular neurodegeneration has never been more critical.
What is Molecular Neurodegeneration?
At its core, molecular neurodegeneration explores how genetic mutations, protein misfolding, mitochondrial dysfunction, oxidative stress, and impaired cellular communication contribute to the gradual deterioration of brain function. It involves identifying molecular pathways and cellular processes that lead to neuronal injury and death. This branch of neuroscience merges molecular biology, genetics, pharmacology, and systems neuroscience to uncover the root causes of neurological decline.
Key Mechanisms in Neurodegeneration
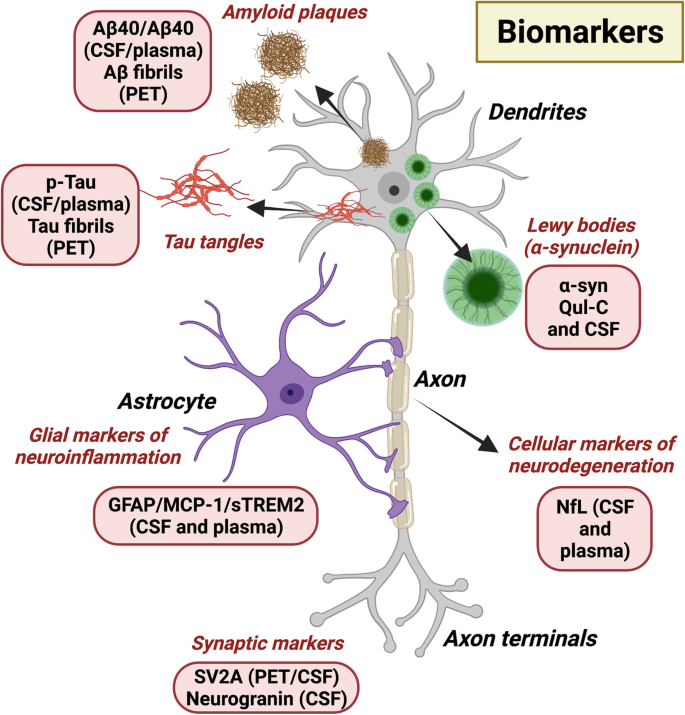
One of the most studied features of molecular neurodegeneration is the accumulation of misfolded proteins. For instance, beta-amyloid plaques and tau tangles are hallmarks of Alzheimer's disease, while alpha-synuclein aggregates are commonly seen in Parkinson’s disease. These toxic proteins interfere with synaptic function, disrupt cellular homeostasis, and eventually lead to neuron death.
Another critical aspect is mitochondrial dysfunction. Neurons rely heavily on energy, and any impairment in mitochondrial activity can lead to cell damage. Oxidative stress, resulting from an imbalance between free radicals and antioxidants, also plays a major role in accelerating neuronal damage.
In addition, neuroinflammation—a chronic inflammatory response within the brain—is increasingly recognized as a contributor to disease progression. Microglial activation, though initially protective, can become detrimental over time, exacerbating neuronal injury.
Genetic and Environmental Factors
Genetics play a significant role in many neurodegenerative diseases. Mutations in genes such as APP, PSEN1, and PSEN2 in Alzheimer’s, or LRRK2 and SNCA in Parkinson’s, can predispose individuals to earlier onset and more severe disease. However, environmental factors such as exposure to toxins, lifestyle, and diet also influence the risk and progression of neurodegeneration.
Emerging Therapies and Research
Advances in molecular neurodegeneration research have opened new avenues for therapy. Targeting specific molecular pathways—such as amyloid-beta production or tau phosphorylation—has become a central strategy in drug development. Gene therapy, RNA-based treatments, and personalized medicine approaches are also showing promise in clinical trials.
Moreover, neuroimaging and biomarker discovery are revolutionizing early diagnosis and disease monitoring, offering hope for intervention before irreversible damage occurs.
About
Molecular neurodegeneration is a critical area of neuroscience that focuses on understanding the molecular and cellular processes that lead to the progressive loss of neurons in the brain and nervous system. This scientific field plays a key role in decoding the underlying mechanisms of debilitating neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and Amyotrophic Lateral Sclerosis (ALS).
As populations age globally, the incidence of these conditions is rising, making research into molecular neurodegeneration more vital than ever. By studying how neurons die at a microscopic level, scientists aim to develop new treatments that can slow, prevent, or even reverse brain degeneration.
What Is Molecular Neurodegeneration?
Molecular neurodegeneration refers to the study of how specific molecular pathways and cellular dysfunctions contribute to neuronal damage and death. This includes protein misfolding, mitochondrial failure, oxidative stress, and neuroinflammation. These cellular events can disrupt normal brain function, leading to cognitive decline, memory loss, and motor dysfunction.
The goal is to identify the root causes of neuronal deterioration and design therapeutic strategies that can intervene at an early stage—ideally before symptoms become irreversible.
Common Molecular Mechanisms in Neurodegenerative Diseases
One of the key contributors to molecular neurodegeneration is the accumulation of abnormal proteins in the brain. For example:
-
In Alzheimer’s disease, amyloid-beta plaques and tau tangles disrupt neural communication.
-
In Parkinson’s disease, clumps of alpha-synuclein proteins (called Lewy bodies) damage dopamine-producing neurons.
-
In ALS, faulty RNA processing and toxic protein accumulation lead to motor neuron death.
Other major mechanisms include:
-
Mitochondrial dysfunction: Neurons require a high amount of energy. When mitochondria fail, neurons lose power and die.
-
Oxidative stress: An imbalance between free radicals and antioxidants causes damage to DNA, proteins, and lipids.
-
Neuroinflammation: Overactive microglia (immune cells in the brain) can lead to chronic inflammation, harming healthy brain tissue.
Genetic and Environmental Influences
While many neurodegenerative conditions have a genetic component, such as mutations in APP, SNCA, or HTT genes, environmental factors also play a significant role. Pesticide exposure, head trauma, poor diet, and a sedentary lifestyle can increase the risk of developing these disorders.
The Future of Neurodegenerative Disease Research
Breakthroughs in molecular neurodegeneration research are paving the way for innovative treatments. Scientists are exploring gene therapy, RNA-based therapies, monoclonal antibodies, and CRISPR gene editing to target disease-causing mutations and pathological proteins.
Early detection tools, such as advanced neuroimaging and fluid biomarkers, are also improving diagnosis and monitoring, allowing for earlier and more effective interventions.
Scope
Molecular neurodegeneration is an evolving field that delves into the intricate molecular mechanisms driving the progressive loss of neurons in the brain and spinal cord. It forms the foundation of our understanding of major neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and Amyotrophic Lateral Sclerosis (ALS). As these conditions continue to rise in prevalence due to aging populations worldwide, the scope of molecular neurodegeneration research becomes increasingly vital.
What is Molecular Neurodegeneration?
Molecular neurodegeneration examines how changes at the molecular level—such as genetic mutations, protein aggregation, oxidative damage, and mitochondrial dysfunction—lead to the degeneration of neurons. These processes often begin years before clinical symptoms appear, making early molecular research critical for prevention and treatment.
The primary aim is to understand how neurons become damaged or die and to identify the molecular targets that can be used to develop therapies for slowing or stopping disease progression.
Expanding Scope in Disease Understanding
One of the most significant areas where molecular neurodegeneration has impact is in unraveling the pathophysiology of brain diseases. The accumulation of misfolded proteins—such as amyloid-beta, tau, and alpha-synuclein—has been closely studied. These toxic proteins impair cell communication and lead to synaptic loss and neuronal death.
This field also explores the role of genetics and epigenetics. Mutations in genes like APP, PSEN1, LRRK2, and HTT have been linked to various forms of inherited neurodegenerative diseases. Understanding these mutations helps researchers develop gene-based diagnostics and targeted therapies.
Interdisciplinary Research Opportunities
The scope of molecular neurodegeneration extends across various scientific disciplines, including molecular biology, genetics, biochemistry, pharmacology, and bioinformatics. This interdisciplinary nature allows for a holistic approach to studying brain diseases, facilitating the development of innovative treatments.
For example, advances in neuroimaging and biomarker discovery are being used to detect molecular changes in the brain before symptoms arise. These technologies provide valuable tools for early diagnosis, patient monitoring, and evaluating therapeutic responses.
Drug Discovery and Therapeutics
One of the most promising aspects of this field is its role in drug discovery. By understanding the molecular basis of neurodegeneration, researchers can identify specific targets for drug development. Current research is exploring:
-
Monoclonal antibodies to clear misfolded proteins.
-
Gene therapies to correct or silence faulty genes.
-
Small molecule drugs to enhance mitochondrial function and reduce oxidative stress.
-
Anti-inflammatory agents to mitigate neuroinflammation.
Personalized medicine is also emerging as a result of molecular insights, enabling tailored treatments based on an individual’s genetic profile and disease biomarkers.
Global Health and Future Prospects
The scope of molecular neurodegeneration extends beyond the laboratory. It has direct implications for public health policy, aging research, and neurological care strategies. As researchers continue to explore this field, the potential to develop preventative strategies, early intervention tools, and curative therapies becomes more attainable.